GOVERNANCE & COMPLIANCE
In the growing regulatory environment with increased focus on Quality, Data integrity to achieve Quality by Design have led organizations to pursue a broad range of governance and compliance initiatives. Synthesis team of Specialists comes with a wide experience to support Lifescience organizations in enabling Governance & Compliance in the GxP areas.
Computer System Validation
Validation of computerized systems according to GxP Guidelines (CSV) has been a basic requirement in the life science industry for many years.
Commissioning and Qualification
Our Mission is to help Lifescience clients ensure their facility, utilities, and equipment perform as intended.
Quality Assurance services
Synthesis Lifescience Specialists assists clients with developing compliant, quality systems that are easy to monitor and control.
21 CFR part 11
Synthesis 21 CFR Part 11 assessment and remediation services are designed to help clients ensure that electronic records are trustworthy.
Early Detection / Audit & Remediation
Pharmaceuticals must be produced consistently and must be strictly controlled to meet both national and international standards appropriate for their intended use.
Computer System Validation
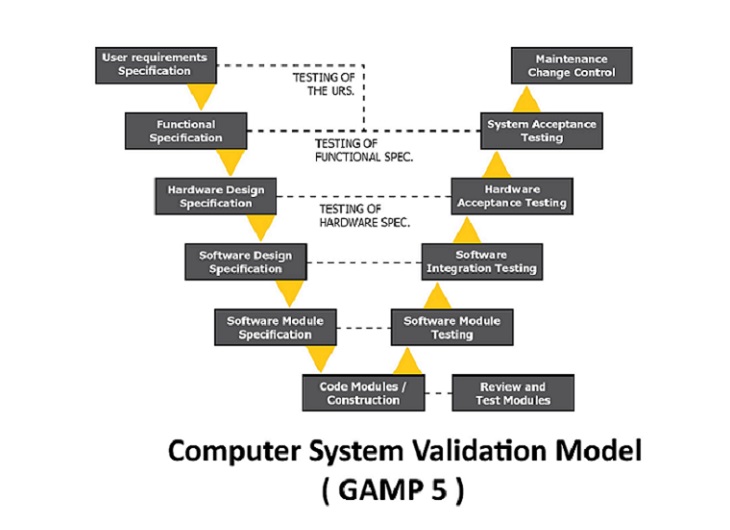
Validation of computerized systems according to GxP Guidelines (CSV) has been a basic requirement in the life science industry for many years. However, as the complexity in the IT landscape and the degree of automation has increased significantly. Synthesis Computer System Validation (CSV) team has significant experience and is a preferred choice for many global pharmaceutical organizations in supporting their application related validation and change management regulatory requirements. Our CSV experts help in analysis, design, execute and manage validation requirements as per the FDA, EU and various global compliance requirements.
We have delivered several Independent Verification & Validation projects utilizing industry best practices. Our CSV services include:
- Independent and/or full validation services for implementations, Data Migrations, Systems Migrations, Application Integrations
- Risk assessment-based validation standards built on industry best practices like GAMP v5
- Ready usable/updateable Validation kits for regulatory compliant applications, data migrations and most common system integrations (e.g. ERP, LIMS, QMS, etc.,)
- Global delivery Model of local and offshore enabling rapid deployment and delivering greater value
Commissioning and Qualification

Our Mission is to help Lifescience clients ensure their facility, utilities, and equipment perform as intended. We do that through commissioning and qualification services, using a risk-based approach to maximize time and cost efficiencies through focused qualification efforts.
Our C&Q specialists has the relevant technical skills and experience to make our clients' projects successful. By using current industry trends and methods to help define and implement best practices, we quickly adapt to the needs of each individual client, knowing each project has unique requirements.
- Manufacturing and Packaging Equipment Qualification (IQ,OQ,PQ)
- Design Qualification (DQ)
- Maintenance System Qualification
- Utilities Qualification
- Facilities Qualification
- FAT / SAT
- Risk Assessment and Risk Management: Direct vs. Non-Direct Impact; Critical Vs. Non-Critical
- Commissioning and Enhanced Commissioning
- Decommissioning
- Integrated Validation Approach

Risk Based Approach
- Interpretation and Application of Quality Risk Management Guidelines (ASTM, ISO)
- Leverage testing from Commissioning (Enhanced Commissioning)
- Eliminate redundant paperwork during qualification
- Reduce test cases based on risk assessments
- Interpretation and Application of new standards based on Risk Based Approach
- Streamline practices and procedures
- Develop risk management exercises (e.g., FMEA)
- Synthesis can conduct on-site supplier audits on your behalf to ensure that their manufacturing activities are being executed according to your specifications and the applicable regulations
Quality Assurance Services

Synthesis Lifescience Specialists assists clients with developing compliant, quality systems that are easy to monitor and control. The importance of regulatory compliance and quality assurance in FDA regulated industry, EU MHRA, and other global regulatory agencies expectations are of paramount. Our team of quality experts assists our clients in Pharmaceutical, Biotechnology, Medical Device, Diagnostic and Active Pharmaceutical Ingredients (API) industry with developing, implementing and maintaining compliant quality systems that are tailored to our clients' specific needs.
Our SME's with extensive experience work in authoring the Quality system processes, Quality Manual, Process SOP's, VMPs in line with the contemporary global regulatory expectation which fits the clients' process.
21 CFR Part 11

Synthesis 21 CFR Part 11 assessment and remediation services are designed to help clients ensure that
electronic records and electronic signatures are trustworthy, reliable, generally equivalent substitutes for paper
records and traditional handwritten signatures and that those functions are in conformance with the
requirements of 21 CFR Part 11 compliance.
Our experienced professionals are extensively trained to evaluate a client's use and documentation of electronic
records and electronic signatures as governed by applicable regulatory requirements. The result of a 21 CFR Part
11 assessment determines the effectiveness of a client's process within a highly regulated environment and
suggests appropriate remedial actions as necessary.
How do we do
Our 21 CFR Part 11 assessments focus on six critical areas:
- Impact of 21 CFR Part 11 on the client's computer systems
- Identification of the client's computer systems and operating environment
- Hosting and interpretation of user interviews
- Review and consideration of client procedures
- Analysis of procedural documentation, validation and audit data
- Regulatory significance of the computer systems

With these six areas being the focal point of our assessment, Synthesis is able to give our client’s an in-depth and thorough inspection of their systems and procedures which is unmatched. We ensure compliance with the requirements of 21 CFR Part 11 through our years of experience and solutions that are unique to you and your company's needs.
Early Detection / Audit & Remediation

Pharmaceuticals must be produced consistently and must be strictly controlled to meet both national and international standards appropriate for their intended use. Strict regulatory requirements must be met including those specified by US Food and Drug Administration (FDA), UK Medicines and Healthcare Products Regulatory Authority (MHRA), other Global Regulatory agencies.
Our auditing team offers Good Manufacturing Practice (GMP), Good Laboratory Practice (GLP), Good Documentation Practice (GDP), Good Engineering Practice (GEP) audit services helping our clients to ensure that all relevant regulatory requirements (FDA, NF, MHRA) are met.
Synthesis can add value to your business by helping you to achieve or maintain regulatory compliance for equipment, facilities, utilities, processes and process installations. Our global network of experienced auditors are available to conduct audits supporting the entire manufacturing supply chain.






